How to Determine Limiting Reactant
After 108 grams of H 2 O forms the reaction stops. Because atoms molecules and ions react with each other according to molar ratios youll also encounter stoichiometry problems that ask you to identify the limiting reactant or any reactant that is present in excess.
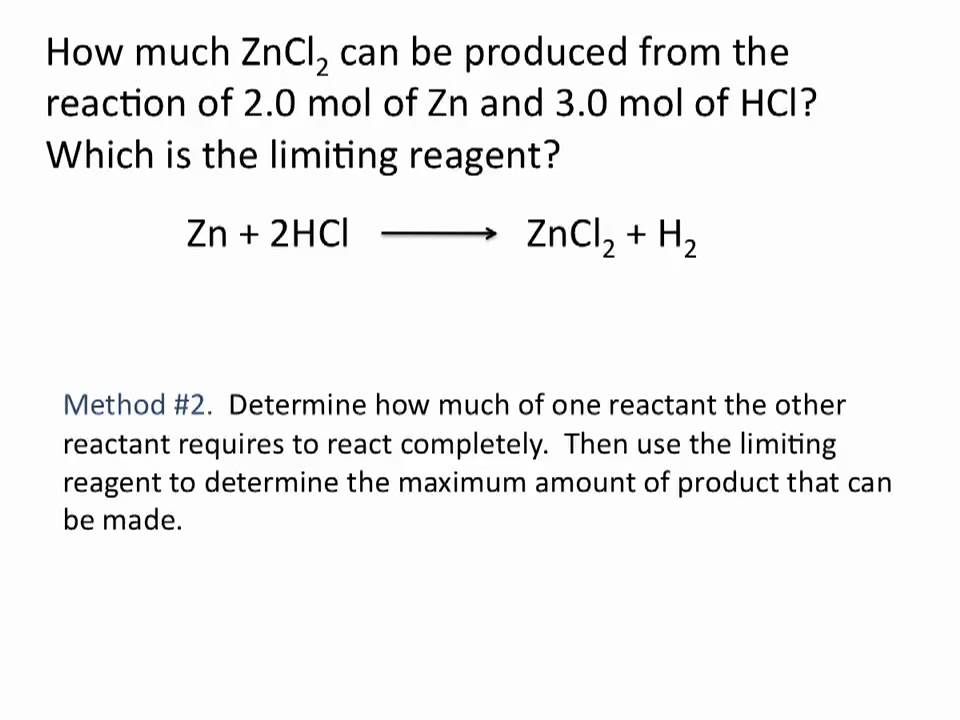
Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial Science
153 g of H2.

. Grams H 2 108 grams H 2 O x 1 mol H 2 O18 grams H 2 O x 1 mol H 2 1 mol H 2 O x 2. 3 Cs Bi2O3s 2. Using the product approach.
Given the following reaction. Limiting Reagent Worksheet 1 1. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button.
The second reflects the fact that anything consumed in the reaction is a reactant not a catalyst. 23 g of sodium metal is transferred to a 3L flask filled with chlorine gas. Literature guides Concept explainers Writing guide Popular textbooks Popular high school textbooks Popular.
Because catalysts are not consumed in the reaction they can catalyze the reaction over and over again. This allows you to see which reactant runs out first. Determine the limiting reagent if 100 g of ammonia and 100 g of oxygen are present at the beginning of the reaction.
First week only 699. You can start with either reactant and. The reactants and products along with their coefficients will appear above.
The Balanced equation is. The fourth criterion results from the fact that catalysts speed up the rates of the forward and reverse reactions equally so the. This calculator will determine the limiting reagent of a reaction.
A Sandwich-Making Analogy This video from Noel Pauller uses the analogy of making sandwiches. PhET sims are based on extensive education research and engage students through an intuitive game-like environment where students learn through exploration and discovery. Determine the reactant which gives less quantity of products and that is called a limiting agent.
In this article How to find limiting reactant the detailed. Limiting reactant are those compounds which are totally used up after completion of the chemical reaction and stop any further reaction. 552 g of HCl Grams of hydrogen formed.
The third criterion is a consequence of the second. Excess Reactant Limiting Reactant and Theoretical Yield. Once you know how many moles of each reactant you have you compare.
The limiting reagent will be highlighted in red. Much more water is formed from 20 grams of H 2 than 96 grams of O 2Oxygen is the limiting reactant. The general problem Given the chemical equation and the masses of reactants determine the mass of.
Assume the following reaction was completed in the laboratory. The limiting reactant or reagent can be determined by two methods. Determine the limiting reagent and amount of excess reagent present if the mass of Na 23 and Cl 355.
To determine the amount of excess H 2 remaining calculate how much H 2 is needed to produce 108 grams of H 2 O. Balance the equation first C 3H 8 O 2----- CO 2 H 2O a If you start with 148 g of C 3H 8 and 344 g of O 2 determine the limiting reagent b determine the number of moles of carbon dioxide produced c determine the number of grams of H 2O produced. One is limiting reactant and another one is excess reagent which left excess after ending the reaction.
To find the limiting reactant you simply need to perform a mass-to-mass gram-to-gram calculation from one reactant to the other. Using the mole ration. Solution for Determine half-life order and k from concentration-time data.
In order to calculate the mass of the product first write the balanced equation and find out which reagent. The following data were collected for the gas phase decomposition of dimethyl ether Skip to main content. In most of the chemical reaction two types of reactant are present.
Start your trial now. In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture. Determine the limiting reactant and calculate the number of grams of hydrogen H2 that can be formed when 453 g of aluminum Al reacts with 552 g of hydrochloric acid HCl.
2Als 6HClaq 2AlCl3aq 3H2g Limiting reactant. Enter any known value for each reactant. Once you have identified the limiting reactant you calculate how much of the other reactant it must have reacted with and subtract from the original amount.
Founded in 2002 by Nobel Laureate Carl Wieman the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations.

Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube Ch 8 Teaching Chemistry Chemistry Notes Chemistry

Skillbuilder 8 5 Unit 1 Stoichiometry Handwriting Worksheets For Kindergarten Persuasive Writing Prompts Chemistry Worksheets

Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial Science

Skillbuilder 8 6 Chemistry Worksheets Scientific Notation Word Problems Chemistry Education
No comments for "How to Determine Limiting Reactant"
Post a Comment